-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Thai Yeng*
Corresponding Author: Thai Yeng, Specialist endodontist, BDS, MDentSci, DClinDent, PhD, MRACDS (Endo), MRCPS (Glasg), FDSRCPS (Glasg), FDSRCS (Edin), FDSRCS (England), FPFA, North Sydney Dental Specialists, NSW, Australia.
Received: September 20, 2023 ; Revised: September 25, 2023 ; Accepted: September 28, 2023 ; Available Online: October 04, 2023
Citation: Yeng T. (2023) The Influence of the Pulp on the Periodontium: A Viewpoint. J Oral Health Dent Res, 3(3): 1-10.
Copyrights: ©2023 Yeng T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
There is an intimate relationship between the periodontal tissues and the pulp-dentine complex, each of which is susceptible to pathological change. A pulpal infection may contribute to periodontal involvement in some cases and vice versa. The type of treatment plan to be implemented will depend on whether the lesion is primarily endodontic or periodontal. In the event of pulpal infection, endodontic treatment should take place first, followed by periodontal therapy, before further review is undertaken to assess the outcome.
Clinical relevance: For a restoration to survive, the periodontium and pulp must be healthy so that the teeth are maintained properly in the arch, which requires the establishment of healthy periodontal tissue.
Aim: The purpose of this article is to illustrate how pulp disease may affect the periodontium, and why endodontic treatment should be the first treatment of choice prior to periodontal treatment.
Keywords: Diagnosis, Endo-perio lesion, Combined lesion, Endodontics, Periodontium, Periodontal
INTRODUCTION
There is an intimate relationship between the periodontal tissues and the pulp-dentine complex, each of which is susceptible to pathological change. A cross-sectional study of general dentists revealed a lack of knowledge regarding the relationship between endodontics and periodontology (endo-perio) [1]. A pulpal infection may contribute to periodontal involvement in some cases. While diagnosing endodontic and periodontal diseases can often be challenging, it is critical to diagnose endodontic and periodontal diseases correctly before providing treatment [2]. Anatomically, when periodontal disease is present, inflammation can pass between the pulp and periodontium at the root apex and through lateral and accessory canals [3]. A third communication pathway between the pulp and periodontal ligament (PDL) is exposed dentinal tubules in areas of denuded cementum [3].
While the apical foramen is the most direct communication route between pulpal and periodontal tissues [4] lateral and accessory canals can also connect the two [5]. These canals may be present anywhere along the root but mainly occur in the apical area and in the furcation of molars. The connective tissue and vessels within the canals connect the circulatory system of the pulp with that of the periodontium [4], providing potential pathways for the spread of bacteria and toxins when inflammation occurs in the PDL [6].
Necrosis of the pulp leads to inflammation of the PDL [1], which results in the opening of the apical foramen and accessory canals. If the endodontic problem is left unresolved for a period, a periodontal breakdown can result [2]. Five types of endo-perio lesions are known: (1) primary pulpal lesions; (2) primary pulpal lesions, accompanied by secondary periodontal disease; (3) primary periodontal lesions; (4) primary periodontal lesions with secondary imposition of pulpal disease; and (5) pulpal-periodontal lesions involving two tissues that have joined or coalesced in the periradicular tissues [7].
AIM
The aim of this paper is to show how pulp conditions, in particular, may impact the periodontium by summarising the following topics: 1) Intercommunication between pulpal and periodontal tissues; 2) Influence of pulpal pathologic conditions on the periodontium; 3) Perforations; 4) Root fracture; and 5) Root resorption.
INTERCOMMUNICATION BETWEEN PULPAL AND PERIODONTAL TISSUE
Three main pathways have been implicated in the development of endo-perio lesions, namely: 1) the apical foramen; 2) the lateral and accessory canals; and 3) the dentinal tubules.
Pulpal and periodontal tissues communicate most directly through the apical foramen [8]. Bacterial and inflammatory by-products may pass through the apical foramen, leading to periapical pathosis and trigger inflammation in the periapical tissues and bone loss [7] (Figure 1).
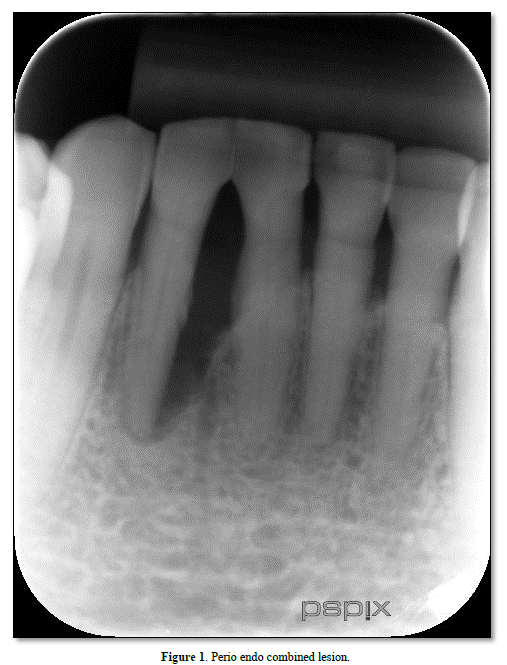
Lateral and accessary canals may appear anywhere along the root, usually in the apical and molar furcation areas [5,8], and patent accessory canals can allow the spread of bacteria and toxins leading to inflammation and widening of the PDL [6]; (Figure 1) Gutman [9] stained 102 molar teeth in a vacuum chamber using safranin dye and found 28.4% of the teeth possessed furcation canals, although only 10.2% did so on the lateral root surface. An analysis by Rubach [10] of 74 teeth revealed that 45% had accessory canals present, primarily near the apex.
Gutmann [9] and others [7] have suggested that dentinal tubules provide a communication pathway between the pulp chamber and external root surface, especially when the cementum is absent. Causes of exposed dentinal tubules in denuded cementum areas include developmental defects, periodontal disease, and periodontal procedures [8]. A study by Ehnevid [11] found that significantly more resorption occurred on exposed dentine surfaces in infected roots compared to non-infected roots. This suggests that endodontic pathogens in root canals appear capable of aggravating periodontal infection in areas of the root devoid of cementum making it is conceivable that dentinal tubules can act as a pathway for bacterial dissemination [12].
Influence of pulpal pathologic condition on the periodontium Möller [13] showed that only pulps that had been devitalized and were uninfected did not suffer periapical bone infection. Moreover, Kakehashi [14] classic study showed that when the pulp of germ-free and conventional rats with natural microbial flora is exposed, only those rats with microbial flora in the oral cavity developed necrosis. Thus, if intra-radicular root canal infection is unresolved, endodontic pathogen growth could be supported and progress into the periodontium via the apex, lateral canals, or accessory canals [7]. The chronic periapical lesions may then drain coronally through the PDL into the gingival sulcus, mimicking a periodontal abscess (Figure 2) [8]. As Figure 3 shows, this differs from a primary periodontal/secondary endodontic lesion, wherein the periodontal pocket extends along the root surface to communicate with the apical foramina or lateral canals [6].
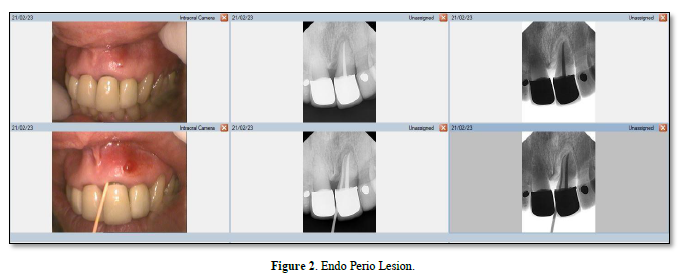
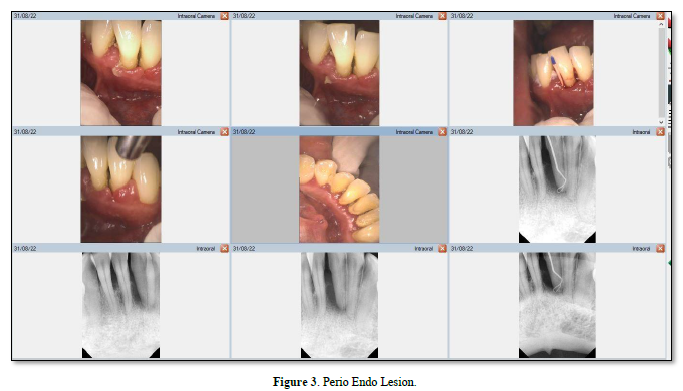
In a retrospective longitudinal study, it was observed that teeth with active periapical lesions in periodontitis-prone patients had a marginal proximal bone loss of 0.19 mm/year, while teeth with no lesions or reduced lesion size had a loss of 0.06 mm/year [15]. Periapical pathology has been shown to contribute to pocket depth formation on periodontal instrumented root surfaces [16]. Following nonsurgical treatment for periodontal pockets exceeding 2.5mm in horizontal marginal defects, researchers found that teeth with periapical pathology were significantly impaired in their ability to reduce mean pocket depth. In contrast, Miyashita [17] failed to demonstrate that diminished marginal bone support is associated with endodontic disease.
Blomlöf [18] reported that aggressive periodontitis and underlying cementum removal adversely affect periodontal healing. Those roots that were not vigorously debrided healed unremarkably. The researchers recommended that definitive periodontal treatment should be preceded by complete endodontic therapy. Periodontal disease of endodontic origin can be healed with proper endodontic treatment [7]. Once root canal treatment is completed for an infection of pulpal origin, endodontic disease will heal, and any suppuration that extends into the gingival sulcus or furcation area and drains through the PDL area will disappear [8]. Treatment should be evaluated in 1 - 2 months to allow sufficient time to elapse to ensure resolution of active endodontic disease [19].
Thus, the recommended approach for endo-perio lesions is to begin with endodontic therapy for primary endodontic disease with associated periodontal involvement [8]. The endodontic treatment and the antibacterial effects of the root canal medicament may affect the intraradicular bacteria in areas where pulpal and periodontal tissues communicate with each other [20], and this may have a positive influence on the health of the periodontium [6].
Root perforations
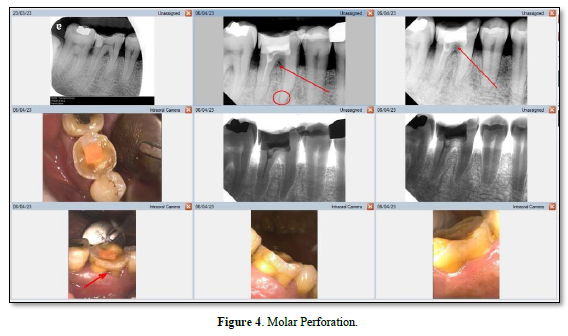
A perforation can result from pathologic or iatrogenic communication between adjacent periodontal tissues and the root canal system [21]. The prognosis for a perforation site depends on whether bacterial infections are prevented or treated [22]. Beavers et al. [23] suggest a periodontal lesion might occur if the perforation is close to the gingival sulcus, causing the gingival epithelium to migrate. The following symptoms may develop if the perforation site is left untreated: a presence of pus exudate, percussion sensitivity of the involved tooth, chronic gingival inflammation resulting from penetration of the alveolar bone, sinus tract formation, or the appearance of localized problems such as periodontal pocket formation or furcation involvement (Figure 4) [24]. Irritants from the periodontal pockets contribute to the perforation inflammation [25]. Perforation caused by a procedural error or mishap. Figure 5 is best referred to an endodontist for immediate management [26] because any delay in treatment will impact the surrounding periodontal health of the tooth and affect the overall prognosis.
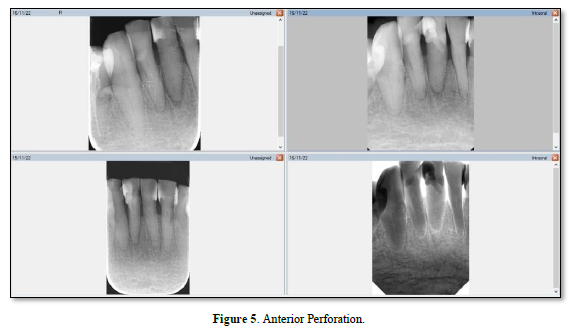
Perforation management aims to promote regeneration of healthy periodontal tissues around the perforation with no lasting inflammation or loss of periodontal attachment [27]. A nonsurgical approach is indicated if the attachment apparatus is intact and there is no pocketing at the perforation site [21]. If there is periodontal breakdown and attachment loss, interdisciplinary consultations (including periodontics, orthodontics and restorative dentistry) should take place [4]. Interestingly, Asgary [28] reported a case that despite negative prognostic factors-sinus tracts, increased probing depth, and radiolucent lesions associated with perforation- nonsurgical repair of the perforation resulted in healing.
Adequate disinfection of the site and repair material during perforation repair is essential for a successful seal, which prevents leakage of irritants and tissue fluid movement into the root canal [29]. Materials such as amalgam, super-EBA cement, bonded composite restoratives, calcium phosphate cement, and mineral trioxide aggregate (MTA) have been used to repair perforations [27]. Besides, possessing many of the ideal sealing properties, MTA has also been recommended as a treatment for root perforations [30] as its high degree of biocompatibility aids regenerative periodontal attachment [31]. Biodentine, another type of bioactive material that has been formulated using MTA-based cement technology, showed lesser bacterial leakage compared to MTA for furcation perforation repair [32]. To place repair material in crestal perforations usually requires a flap, since they are close to the dentogingival junction [29]. Surgery is usually reserved for defects that cannot be treated with other methods [24].
A marked difference in healing rates has been reported between root canal treated teeth with and without preoperative perforation. Gorni [33] and Farzeneh [34] both reported similar success rates around 86% for teeth without perforation. This compares with a very low success rate of 36% for teeth with perforations [34]. Main [31] reported the use of MTA to repair root perforation in 16 cases. Radiographic follow-ups at least one year after the MTA repair revealed complete healing in all cases. Initially, the risk of reversal for healed MTA-treated root canal perforations is relatively low, in the longer term the risk increases dramatically. The estimated reversal probability at 5, 10, and 14 years is 6%, 30%, and 62%, respectively [35]. This suggests the prognosis and long-term outcome of teeth with perforation is uncertain compared to those without.
Root fracture
A root fracture can also present as a primary endodontic lesion associated with periodontal disease [36]. A periodontal pocket may deepen locally or develop into an abscess, depending on the severity of the infection [37]. Long-term root fractures are often associated with PDL irritation, resulting in alveolar bone destruction [37]. Vertical root fractures (VRF) can cause progressive periodontal destruction, despite an overall stable periodontal state and proper endodontic treatment [38]. This is illustrated in Figure 6.
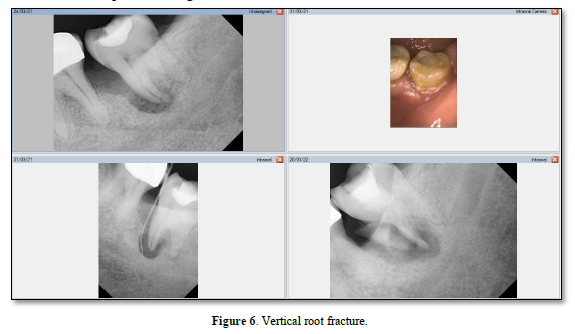
Maxillary premolars are prone to fracture, while maxillary second premolars and mandibular molars often fracture [39]. Viré [40] found that horizontal root fractures occurred in 4.3% of endodontically treated teeth that were extracted. Root canal-treated teeth may be subjected to greater occlusal forces because they may not have the same proprioceptive abilities as non-endodontically treated teeth [41]. The combination of structural failure, pre-existing fractures, and the biochemical effects of vitality loss can cause a higher prevalence of vertical root fracture in root-filled teeth [42].
Tamse [39] found that 67.4% of endodontically treated teeth with VRFs had a solitary buccal pocket and 34.8% had fistulas, most frequently near the gingival margin rather than the apex. Vertical roots generally fracture in a buccolingual direction, except for maxillary premolars with two roots and maxillary molars with a mesiobuccal root that exhibit more variability in fracture sites [43]. An endodontically treated tooth can also suffer from dull pain and swelling because of a VRF [44]. VRFs of maxillary premolars most often appear as halo lesions (57%), angular bone loss (14%), and periodontal radiolucency (14%) according to Tamse [39]. In rare cases, the PDL space may also appear diffusely enlarged on a radiograph [45]. Yang [46] reported the high accuracy of cone-beam computed tomography (CBCT) in detecting VRF. Overall, root fractures can cause chronic inflammation of the cementum and PDL, resulting in periodontal breakdown and, ultimately, a periodontal pocket36 and late failure in endodontic treatment [47].
Root Resorption
Resorption of the root canal wall inside a tooth is referred to as internal root resorption; and resorption of the external root surface is known as external root resorption [48].
The process of root resorption (RR) requires two conditions. First, the protective layer (precementum or predentine) must be lost or altered; and second, the unprotected root surfaces must be inflamed [49]. Bacterial inflammation can cause compounds such as osteoclast activating factor, prostaglandin, macrophage-chemotaxis factor, and bacterial lipopolysaccharide to stimulate hard tissue resorption [50].
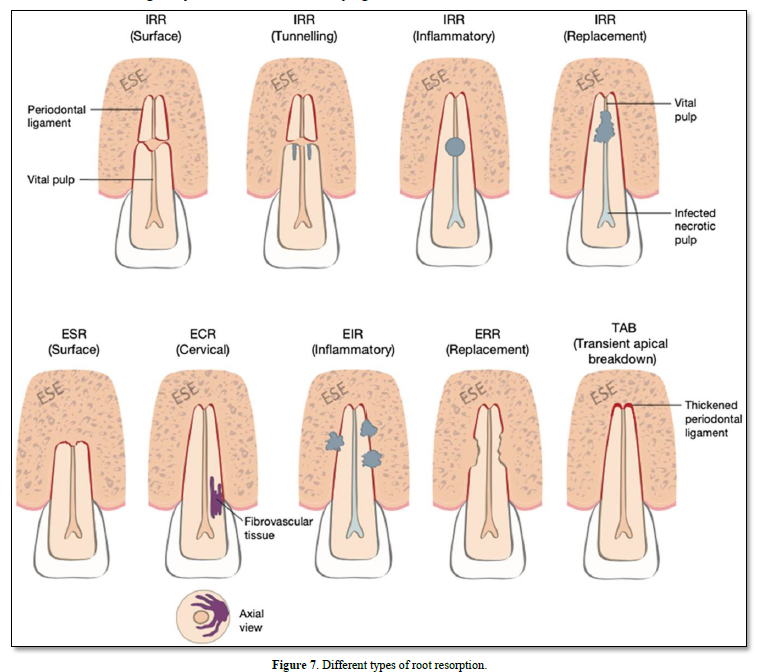
Figure 7 shows the different types of RR that exist [48]. It is difficult, however, to report the prevalence of each type because of the heterogeneity of methods for identifying and measuring them [42]. With few RR circumstances the same, and each with specific treatment options, this paper will select a few examples to demonstrate the impact of RR and how an endodontic infection can affect the periodontium.
During surface resorption, cementoblasts cover damaged root surfaces, and macrophages and osteoclasts resorb the layer of PDL nearest the cementum [48]. Toxins infecting a root canal may move to the PDL after surface resorption penetrates the cementum and exposes dentinal tubules [49]. However, the root surface contour is restored after a few weeks by cementum and fibrous ligament fibers deposited in the resorption cavities [51].
External inflammatory (infection-related) resorption is triggered by the loss of the protective precementum layer on the external root surface, combined with the root canal microbiome of necrotic pulp from an infected tooth [52]. This type of resorption only occurs when the root canal becomes infected (Figure 8) [42].
Inflammatory resorption, demonstrated by Heithersay [53] occurs when the root canal becomes infected with necrotic tissue or a leukocyte zone. When the root loses its cemental protection, it can develop lateral periodontitis with RR [49]. Until endodontic treatment is initiated, the inflammatory RR process continues [54].
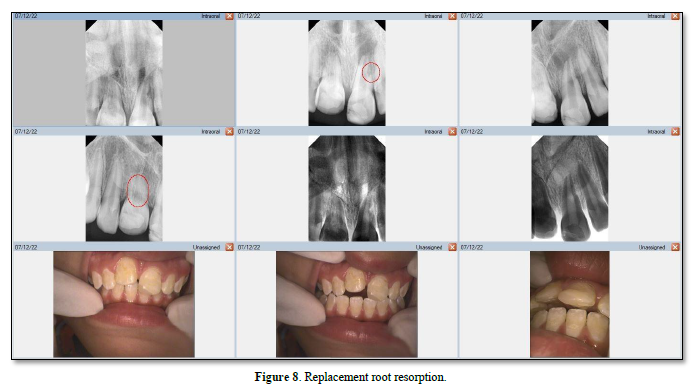
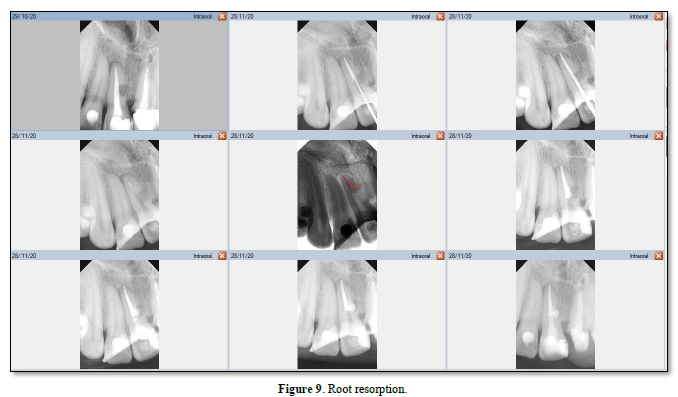
In replacement RR, the root surface lacks a vital PDL covering [55] and necrosis may result from damaged PDL cells that have been torn, crushed, and/or degenerated [48]. Necrosed PDL cells and damaged cementum and dentine are resorbed by the osteoclasts; osteoblasts then generate alveolar bone (Figure 9) [56]. With this type of resorption, bone replaces dentine and/or cementum [49], which is usually associated with ankylosis [57].
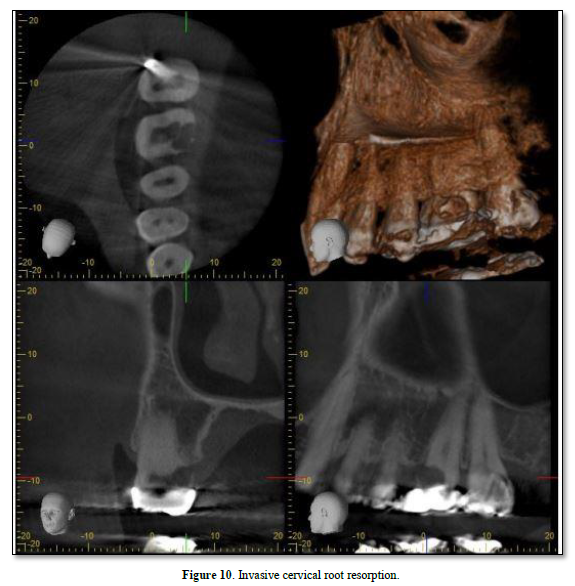
Cervical RR, a relatively uncommon form of external RR, is characterized by its invasive nature and can be detected by CBCT [58]. It tends to discolor the tooth crown giving it a pink hue (Figure 10) [53]. The exact cause of invasive cervical resorption is unknown; however, there is a strong correlation between orthodontic treatment, trauma, and intracoronal bleaching [59]. This type of resorption occurs below the epithelial attachments of the tooth [48]. As the source of infection is not the pulp, it has been suggested that bacteria in the sulcus stimulate and sustain inflammatory responses in the periodontium [60]. The destruction will continue until either there is no root structure left, or the inflammatory stimulus is removed by intervention [49]. Heithersay [61] has classified this type of resorption into four classes of severity with the proposed treatment regimens. Treatment may involve vital pulp therapy [62]; root canal treatment [42]; or surgical management [63], which may include topical trichloroacetic acid application, curettage, bioactive materials (e.g., MTA, Biodentine etc.) [64], and restoration with glass ionomer cement as needed [59].
CONCLUSION
Tooth anatomy reveals multiple pathways for bacteria and their by-products to travel between the pulp and the periodontium. When pulp degeneration products reach the supporting periodontium, they can cause an inflammatory response leading to bone loss, tooth mobility, and sometimes sinus tract formation. Pulpal infection can hinder periodontal healing following periodontal therapy and may also increase radiographic attachment loss and impact the health of the periodontium compared to teeth with a healthy pulp. Based on the evidence, patients with a diagnosis of endodontic-periodontal lesions should initially undergo endodontic treatment before any periodontal treatment.
No Files Found
Share Your Publication :